A “SMART” TESTING STRATEGY TO CONTROL COVID-19 IN CANADA
Vivek Goel, Peter Nicholson, and Jeff Larsen
NOTE TO READERS: A PDF of the full paper with sourced footnotes and tables, as well as a brief biography of the authors[1], is available for download here.
Testing is not an end in itself but is undertaken to inform the decisions needed to control the virus responsible for COVID-19 while minimizing unintended consequences. Our argument, summarized below, is that Canada should be testing a great deal more, as is the case in the majority of countries that have been successfully controlling the virus. But increased testing needs to be smart and targeted strategically because testing resources will never be unlimited, nor are tests perfect.
A testing strategy to control COVID-19 must be tailored to the particular infection characteristics of the SARS-CoV-2 virus—the fact that transmission often occurs from individuals who are contagious but who do not yet display symptoms or who have symptoms so mild that they are unaware. Seeing no reason to isolate themselves, they move about and potentially infect others.
The focus of traditional testing for contagious disease is on those who do present with symptoms, as well as on their close contacts. These individuals can be immediately isolated pending confirmation of infection.
In the case of COVID-19, that approach needs to be extended to quickly identify and isolate the invisible spreaders and their contacts, an approach referred to as “test, trace, and isolate.”
This requires a strategy for “surveillance” testing of groups that are at higher than average risk of exposure to the virus or of transmitting it to particularly vulnerable individuals.
Surveillance requires high volume testing and thus relies on testing technologies that are convenient and inexpensive and that, in many cases, provide rapid results.
Surveillance testing does not replace testing currently being used for diagnostic and related purposes where the accuracy of the test is paramount. Highest accuracy relies on “PCR” technology, regarded as the gold standard for detecting the virus that causes COVID-19.
Surveillance testing, on the other hand, is complementary to diagnostic testing and requires technologies or approaches that are simpler, quicker and cheaper but do not require quite the diagnostic standard of accuracy.
The strategy we propose would employ on the order of at least half a million tests per day across Canada—more than seven times the current testing rate—and would be achieved by significantly expanding existing PCR capacity, augmented for screening purposes with “antigen” tests that, while less accurate, are inexpensive and deliver a result in minutes.
The implementation of this strategy requires, in addition to procurement of much larger testing resources, more widespread recognition by public health authorities of the need for surveillance testing to complement existing diagnostic testing. Both are necessary for optimal control of COVID-19.
The paper is structured as follows: first, a review of several technical aspects of tests intended to detect an active infection by SARS-CoV-2; second, the purposes of testing, which we define as Diagnostic, Screening and Reassurance; and third, considerations relevant to a smart testing strategy to accomplish these purposes.
1.1 The infection process
Infection by SARS-CoV-2 begins with transmission from an already infected person.
The dominant mode of transmission is by “droplet” spread enabled through prolonged close contact with a contagious individual—e.g., within about 2m for at least 15 minutes. Droplets containing the virus are expelled from the mouth (coughing or talking) or nose (sneezing) and typically spread for a maximum radius of approximately two meters. The term droplet may be confusing because a droplet in this context is not necessarily sufficiently large to be seen. Talking, particularly in a loud voice, or singing, generates abundant droplets of sizes that are normally invisible but which can contain the virus and enable it to be inhaled by a person close by. That is why the risk of spread is particularly great in crowded and poorly ventilated indoor environments where people are talking face to face, shouting or singing—e.g., bars, parties, church services, assembly lines, and family and other congregate living spaces. Transmission via droplet can be greatly reduced by physical distancing and mask-wearing.
The virus can also be spread within aerosols—tiny exhaled particles of less than about 5 micrometers in diameter that can remain in the air for long periods and drift far from the original source, depending on air circulation. Although the evidence is not definitive, and there is a divergence of expert opinion, the current majority view of scientists is that aerosol transmission does occur but plays a much smaller role than droplet transmission. That risk can be further reduced through use of masks and good ventilation of indoor spaces—e.g., as provided on commercial airplanes.
There are other channels of transmission—notably when someone touches a surface on which droplets containing the virus have landed, and then touches their mouth, nose or eyes thus giving the virus access to specific types of tissues that it can invade. While there are laboratory-based studies which suggest that the virus may be able to survive on surfaces for many days, clinical and epidemiological evidence implies that it is not likely in real world settings. This type of direct contact transmission is therefore believed to account for only a few percent of infections and can be minimized by good hand hygiene.
The contagious period of COVID-19 typically begins about two days after exposure to the virus with the quantity of the exponentially growing virus peaking usually between days 5 and 7, then declining to the point when the individual is no longer likely to be contagious by days 12-14.[2] It is believed that the severity of the disease correlates with the patient’s viral load as does the likelihood of infecting others. Although the quantitative evidence is mixed, it appears that up to about 40% of infections may be spread by those without symptoms—either because their symptoms have not yet emerged (pre-symptomatic), or because their symptoms are very mild or apparently absent (asymptomatic).[3] Being unaware that they have the disease, these individuals make no particular effort to isolate themselves. The prevalence of asymptomatic and pre-symptomatic transmission is therefore what makes COVID-19 much more difficult to control than previous coronavirus outbreaks like SARS and MERS that were effectively contained because the virus was transmitted predominantly by symptomatic individuals that could be readily identified and isolated. To control COVID-19 we need testing strategies geared to the unique biological characteristics of SARS-CoV-2 transmission.
1.2 Testing for SARS-CoV-2
There are several types of test—both in use and in development—that detect active infection with SARS-CoV-2. Our focus here will be on these tests and their appropriate use in strategies to minimize the risk of spread.[4] Following is a brief outline of the relevant characteristics of the principal tests currently used to detect active infection.
The PCR Test—This test, considered to be the gold-standard, uses what is called the polymerase chain reaction (PCR). This process amplifies, to a detectable amount, the virus’s genetic material (RNA) in a sample that is typically obtained through a nasopharyngeal swab. Less invasive methods include a throat swab or back-of-nose swab. Although generally collected by a healthcare professional, procedures for self-collection, including of nasal/oral swabs and saliva samples, are now becoming more widely available. Self-collection has the potential to significantly increase the convenience and decrease the cost of PCR testing although with some extra risk that samples will not be collected properly.
PCR tests require analysis primarily in central, specialized labs with trained personnel and rigorous protocols for quality assurance, tracking and communication of results.
Although PCR tests are the most accurate currently available, their disadvantage is the complexity and cost of overall administration and the time to produce a result—typically 12 to 24 hours, provided there is little backlog. The total time from sample collection to reported result includes the time to transmit the patient sample from the test location to the central lab; the lab process itself (typically 6 to 12 hours, of which the RNA amplification and read-out takes about 3.5 hours); and finally the time to have the result logged and reported. Thus the accuracy of lab-based PCR testing comes at a cost of time, convenience and resources, and capacity can be limited by shortages of materials, personnel or budget. Across the country combinations of public health, hospital and private labs provide the services, often working with different methods, equipment and information systems. This diversity of approaches decreases the efficiency of PCR testing, reduces timeliness, and increases costs and inconvenience. As many labs are using equipment that require specific proprietary test kits, they are often also limited in capacity because of supply chain challenges. (Approaches to improving efficiency, timeliness, and expense of PCR tests are discussed later.)
Rapid Tests—The ideal alternative to the standard PCR test would be a much quicker test, preferably simpler to process and less expensive. There are several such tests in use and in advanced development. Some require lab processing, while the majority can be processed at the point of care (“PoC”).
The most commonly deployed PoC test for COVID-19 in Canada is the GeneXpert from Cepheid Inc. which must be administered by a trained technician and delivers a result within about 45 minutes. The GeneXpert is useful in locations remote from a central PCR lab but it is not designed for high-volume testing. Health Canada has recently authorized a second PoC test, the ID NOW from Abbott Laboratories. The federal government has ordered almost 8 million of these test-kits and 3,800 of the toaster-size devices in which samples are analyzed. The ID NOW delivers a result within less than 15 minutes and is easy to use, although a trained healthcare worker must conduct the test. Both the GeneXpert and ID NOW detect viral RNA but do not amplify it to the same extent as the standard lab-based PCR analyzers and thus the gain in speed and simplicity comes at some cost of lower detection sensitivity.[5]
A promising type of rapid PoC test is an antigen test which detects the presence of viral proteins (antigens) in a biological sample such as saliva or tissue swabbed from the nasal cavity. These tests are typically cheap; might be self-administered at home; and return results in minutes, but do not yet approach the accuracy of PCR. So far four antigen tests for SARS-CoV-2 have received an Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration, but only one has recently received similar approval from Health Canada.
Finally, at the leading edge of viral testing technology are genome sequencing tests which detect the RNA of SARS-CoV-2 from respiratory specimens utilizing next generation sequencing technology. The FDA has so far granted an EUA for at least one such test, but none have been approved in Canada. This technology is likely too immature to play a significant role during the COVID-19 pandemic but may be a revolutionary testing tool for use in future outbreaks of viral diseases that will inevitably occur.
1.3 The interpretation of test results
No test for the virus can be perfectly accurate for the following principal reasons:
Every test has a characteristic lower limit of detection that depends on the amount of virus in the sample. In the case of COVID-19, even a PCR test is unlikely to detect the virus during the first couple of days post-infection.
Error that is inherent to the particular testing technology employed. PCR tests are the most accurate due to their biological properties combined with the capability to detect very small amounts of the virus. But PCR may also detect residual fragments of viral RNA long after the individual is no longer contagious, thus leading to a form of “false positive.”
Improper collection or handling of the sample to be tested or clerical errors in reporting the results. These sources of error can be reduced with care and training, but never completely eliminated in the real world.
The inherent accuracy of a diagnostic test is characterized by two numbers—sensitivity and specificity. The sensitivity of a test is the likelihood that it will register a positive result if the virus is actually present, while specificity is the likelihood that the test will register a negative result if the virus is not present. Both parameters are usually expressed as percentages. The sensitivity and specificity will be higher under controlled lab conditions than in use in the field. Under realistic conditions the sensitivity of PCR tests ranges between about 70% and 95% whereas specificity is much higher, usually approaching 100%. Antigen tests are less sensitive than PCR—estimates vary greatly—but appear to have comparably high specificity although specificity may be reduced by the presence of contaminating proteins.
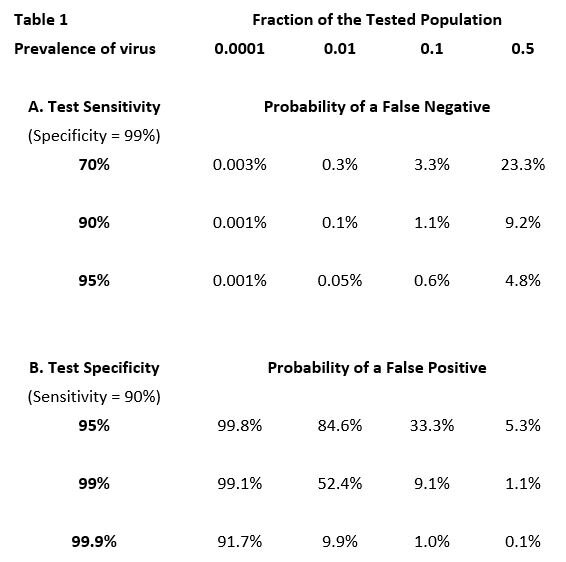
The proportion of negative test results that are false negatives—or of positive results that are false positives—depends not only on the sensitivity and specificity of a particular type of test, but also crucially on the current prevalence of active infection in the population being tested. For example: if there were absolutely no infection in the tested population, every positive result (due to less than perfect specificity) would be a false positive, whereas at the opposite extreme, where everyone is infected, every positive result would be a true positive. More generally, as the prevalence of infection in the tested population increases, the proportion of false positives declines. The proportion of false negatives changes in the opposite direction. When the prevalence of infection is low, the proportion of false negatives—as a fraction of all negative results—is low. For example, if no one in a population is infected, there are no false negatives. But as prevalence increases, so does the percentage of negative results that are false (Table 1)[6].
In the top row of the table the prevalence of the virus ranges from 1/100th of one percent (.0001) of the tested population to 50% (0.5). The entries in the table are the expected percentages of false results (negative or positive) over the typical range of test sensitivity and specificity.
Summarizing—As can be seen in Table 1 below, when the prevalence of infection is low in the tested population, even a test with fairly poor sensitivity will not miss a high proportion of those infected. But unless the test has very high specificity, it will generate a lot of false positives. Fortunately, both PCR tests and antigen tests have inherently high specificity. When the virus is believed to be common in the tested population, high test sensitivity is needed to avoid an unacceptable proportion of false negatives.
The proportion of currently infected persons in the entire Canadian population is still very low despite the recent increase in confirmed cases. For example, at a national rate of 2,000 confirmed cases per day, and assuming that the true infection incidence might be three times the confirmed number, only about 0.2% of the population would be carrying an active, and potentially contagious, infection during any two-week period. Nevertheless, the proportion of positives that are false will be very high at such low prevalence of the virus. Of course, testing is usually undertaken in circumstances where there is reason to believe that the persons being tested have a higher than average likelihood of being infected—e.g., symptoms are present, the individual has been in contact with an infected person, or is involved in activity believed to be higher risk. In these situations, it is important to use tests that have high sensitivity in order to reduce the proportion of false negatives.
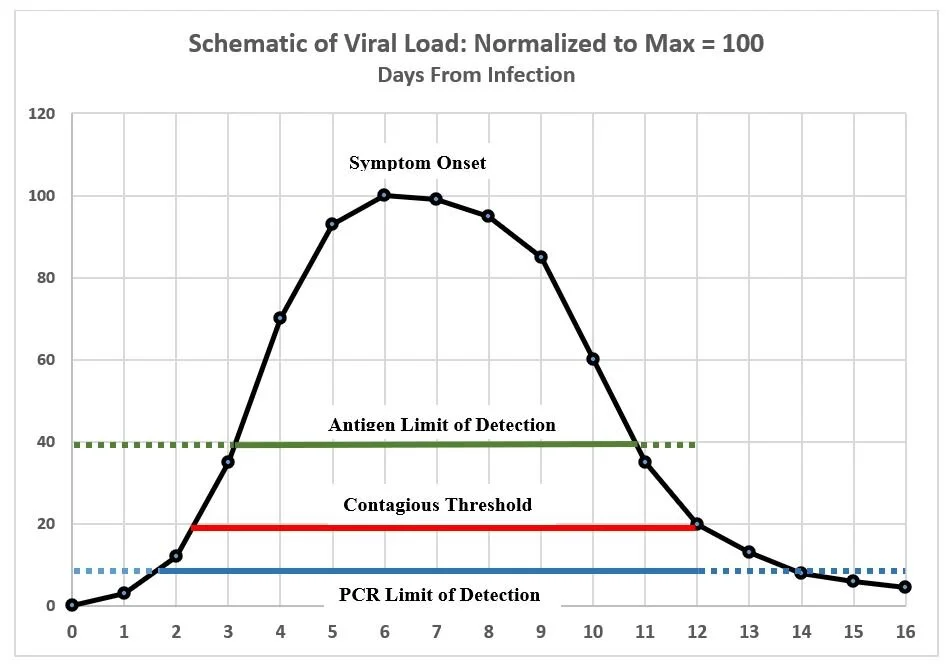
The actual sensitivity and specificity of a test in the “field” depends on the stage of the subject’s infection when the test is administered. This is illustrated schematically in the following chart that compares the hypothetical performance of a PCR and an antigen test relative to the time interval when the subject is likely to be contagious.
This chart is intended only to illustrate certain concepts. While based on various estimates in the current literature, it does not represent actual data which, moreover, varies substantially from case to case. The curve traces the typical course of viral quantity during the progression of COVID-19 from initial infection (Day 0) to resolution, typically in about two weeks. The contagious period usually begins between Days 2 and 3—two to three days before the typical onset of symptoms—and is believed to last for about 10 days. A PCR test usually (i) will return a negative result within 1-2 days after infection but before the individual is contagious (dotted blue line to the left), (ii) will almost always return a positive result during the contagious period, but (iii) will continue to register positive for days or possibly weeks after viable virus has declined to a level where the individual is no longer contagious (dotted blue line to the right). That is because PCR will detect fragments of viral RNA even after live virus has been eliminated—e.g. well beyond Days 14-16 in the schematic chart. A positive result after Day 12 in the chart would in effect be a false positive. The antigen tests developed so far have a significantly higher limit of detection than PCR and thus may: (i) miss roughly the first 3 days of infection, including an initial part of the contagious phase (dotted green line to the left), (ii) detect the virus for the next 7-8 days, covering perhaps 80% of the contagious interval, but (iii) may miss a day or so at the end of the contagious phase (dotted green line to the right.) For antigen tests evaluated so far, it is advised that they be used only within 7 days from onset of symptoms. This of course would not be applicable in asymptomatic cases, whereas it is likely that an antigen test could detect infection during some portion of the contagious period although definitive evidence is lacking as to the probability of detection in such cases.
1.4 The trade-off between accuracy and speed
Clearly the PCR test is more accurate (i.e. sensitive) than available antigen tests. But what matters for control of spread is the sensitivity during the contagious period. In this regard the difference between PCR and antigen is probably less than the sensitivities measured under lab conditions would suggest. The antigen test requires a lot more virus to trigger a positive result, but in the first several days of the infection the viral load is growing very rapidly, thus shrinking the time interval between the possibility of first detection via PCR and via antigen. Moreover, beyond the time when the individual is no longer contagious (beyond Day 12 in the schematic chart) the antigen test is actually more reliable than PCR since the latter may still return a positive result because it is detecting fragments of non-viable virus. For practical purposes this amounts to a false positive. While false negatives are more costly in epidemiological terms—thus putting a premium on sensitivity—false positives are damaging in economic terms since they require isolation pending confirmation and consume resources for re-testing. Minimizing these costs depends on tests of high specificity.
In contact tracing and follow-up testing—which is the key to controlling spread—the challenge is to identify the contacts of a potentially contagious individual and to convince them to be tested. It is obviously important to do so very quickly so that, if they test positive, they can be isolated before exposing many others. This puts a premium on quick turnaround of test results. In view of the need for speed, and the potentially large number of contacts per infected person, a rapid testing capacity is ideal. The availability of a quick turnaround test also makes it more likely that contacts will agree to be tested and self-quarantine pending the result, which for an antigen test is only several minutes. Modelling studies suggest that use of a rapid test may be more effective in limiting spread of infection than a slower PCR despite the latter’s superior accuracy. The foregoing considerations have informed Health Canada’s interim guidance regarding the prospective use of antigen tests.
2.1 The purposes of testing for active infection
We identify three principal purposes for testing—Diagnostic, Screening and Reassurance.
Diagnostic—The first purpose is to test individuals who (a) present symptoms related to COVID-19, or (b) have had recent close contact with a person that is known or suspected of being infected. The primary objective of clinical diagnostic testing is accuracy—thus the virtually exclusive use of PCR technology in this circumstance.
Screening— The second principal purpose is to test individuals who may be at some risk of being carriers of the virus in order to identify contagious individuals that do not display overt symptoms. The objective is to identify and contain these “invisible” carriers before they infect others. Screening testing would not be random, but rather focused on settings, activities, and persons that are believed to be higher risk—e.g., travellers from areas with relatively high infection rates; healthcare environments and other situations where there is frequent contact with vulnerable individuals; residents of homeless shelters and other congregate living arrangements; schools, universities and other work situations, such as meat-packing plants, where extended close contact is unavoidable. Screening testing needs to be an integral part of a national strategy together with the prevailing testing for diagnostic purposes. Screening should not be regarded as optional.
Reassurance—This third purpose refers to testing individuals who have no symptoms and where there is little or no reason to believe that they may be contagious. There is no sharp dividing line between screening and reassurance testing but it is conceptually convenient to divide the very broad spectrum of risk into two qualitative categories. Some testing can be justified simply to reassure the public that COVID-19 is being kept under control. Greater confidence is clearly needed before life can return to something approaching normal. For example, reassurance testing might be undertaken by certain employers to reduce workplace anxiety; or where individuals request a test for peace of mind; or by international travellers to meet the pre-departure requirements of destination countries. In most cases the cost of reassurance testing should be borne by those requesting it although some subsidization may be appropriate depending on need. It could be provided by private organizations or by the public testing system if spare capacity were available. Reassurance testing could use rapid, inexpensive point-of-care (PoC) technology thereby reducing cost and increasing convenience in a situation where the PCR standard of accuracy is not required. In fact, reassurance testing could in some cases provide a trial environment for new test technologies.
There is a distinct fourth category—Surveillance testing—which is undertaken to monitor a population or community for a level of infection or to characterize the incidence and prevalence of a disease. The aggregated information is made available to public health authorities and not to individuals. Examples would be serologic testing of a population to determine the past course of an epidemic; aggregated results of screening and reassurance tests to track the incidence of infection in real time; or testing a community waste water source for presence of SARS-CoV-2. Testing for presence of the virus in sewage can play an important role in the early detection of cases in a population in a demarcated facility like a university residence, long-term care home, factory, prison, homeless shelter or a school. The detection of the virus would be a signal to perform screening testing of the individuals that contributed to the common source of sewage.
We test for a contagious virus to assist decision-making—to initiate treatment; to isolate or quarantine; to interact cautiously with others, or occasionally to throw caution to the wind. Because no test is infallible, the results should always be contextualized. Does the tested individual present symptoms? Is the disease locally prevalent? Has the individual been in close contact with persons known or suspected to be contagious? In any of these circumstances a negative test result should not be taken at face value. The choice of testing frequency and the type of test to be employed will also depend critically on context and particularly, as noted above and quantified in Table 1, on the expected prevalence of the virus in the population being tested and thus the relative importance of the test’s sensitivity and its specificity.
The consequences of a false test result must be interpreted in the light of the “cost” of the error. For example, the consequences of a false negative result—missing a contagious person—when testing a worker in a long-term care home, or a resident in a congregate living setting, would be very serious owing either to vulnerability in the first case or the high risk of spreading the virus in the second. On the other hand, a false positive is costly for a worker who has to be quarantined pending further evaluation as well as for the public health system that has to allocate resources to correct a false result.
Faced with the urgency created by COVID-19, the technology of testing has rapidly advanced to the point where tests that are much faster, cheaper and more convenient than traditional PCR are now becoming available. Meanwhile, the convenience and cost of PCR is also improving—e.g. through the use of saliva sampling instead of swabs, pooling of several samples in situations where the incidence of the virus is low, and use of high-throughput technology. These efficiencies, combined with higher demand, have created a new opportunity for dedicated COVID-19 PCR labs to extend and complement the capacity of the public system in Canada.
Antigen tests—because they are simple, very rapid and inexpensive—have the potential to massively expand testing capacity beyond applications to clinical diagnosis and contact tracing. This class of test increases the feasibility of regularly screening target groups that are of greater risk of spreading the disease or where the consequences of spread are particularly high. The new generation of antigen tests could be deployed cost-efficiently at very high volume with back-up by PCR in situations where reliable confirmation is required. However, at present, the characteristics of these tests in broad testing of asymptomatic populations requires further examination.
3.1 Fundamentals of a testing strategy
Table 2 below illustrates the type of strategy that might be employed to vastly expand testing to screen a variety of target groups to identify pre-symptomatic and asymptomatic individuals. The strategy relies on separating the choice of appropriate test into situations that require: (a) an accurate test despite it being slower and more costly, or (b) a rapid, inexpensive test but at some sacrifice of accuracy. The former situation presently calls for the traditional PCR test although cheaper and quicker tests, with accuracy comparable to PCR, are becoming available. Meanwhile; high throughput PCR with sample pooling has the potential in many circumstances to meet the speed and low-cost thresholds for mass screening and “reassurance” testing.
These considerations imply that the test selection decision needs to be focused on the requirements of the test, and not on the technology of the test per se. For that reason we label the test choices in Table 2 below as ACC for “accurate” despite being relatively slow and expensive—today’s standard PCR—and RNX for “rapid and not expensive” despite being relatively less accurate. The latter would include antigen tests. It is likely that as technology advances some variants of PCR become “RNX” while antigen or other cheap rapid tests become sufficiently “ACC” for the purpose. The test sequences in Table 2 are intended only to be illustrative. The actual decisions would depend on specific factors to be evaluated in context.[7] Implicit in the table is the assumption that RNX tests will be approved in Canada and available in quantity. As antigen tests are deployed globally at high volume it is likely that shortages will develop, making proactive procurement a priority.
Note: The type of test is labelled “ACC” indicating a test of high accuracy like PCR; or “RNX” indicating a test that is rapid and not expensive, like an antigen test or some implementations of PCR.
3.2 Is universal testing feasible, or desirable?
More testing is generally preferred to less, provided the capacity exists to give priority to diagnosis and contract tracing. This raises the question as to whether the goal should be to test every Canadian as frequently possible. To address this question hypothetically, imagine an ideal situation in which there is a test for SARS-CoV-2 that costs a dollar; can be self-administered at home; and that yields a result in less than 15 minutes.[8] A COVID test could then become part of everyone’s morning routine just like brushing your teeth and washing your face.
Suppose for illustration that the test had 75% sensitivity and 97% specificity. Should (almost) every Canadian use it every day, or perhaps every third day, or once a week?[9] The answer will depend on the incidence of true and false results. That in turn will depend, not only on the inherent sensitivity and specificity of the test, but also critically on the prevalence of active infection in the overall Canadian population. Currently about 2,000 new cases of COVID-19 are being confirmed every day, and perhaps as many as twice that number are being infected but are not tested—e.g., because they have no symptoms—implying as many as 6,000 new infections per day. If we assume that a person might be contagious for about 10 days, then on a given day there will likely be about 40,000 contagious people circulating in society—i.e. those infected today and on the previous nine days, less the 2,000 per day who have been identified and isolated. At the end of Day 1 of universal testing, a test of 75% sensitivity will have identified three-quarters of contagious people but missed 25%, or 10,000 individuals in this example. Then on Day 2, three-quarters of those false negatives will be identified and quarantined but a quarter will remain undetected plus a quarter of the new infections that day. As the days pass the universal testing procedure soon reduces the total number of contagious individuals in the population to about 8,000, assuming the number of daily new infections remains constant at 6,000.[10] In fact the number of new infections would decline rapidly under this testing regime and could only be sustained by a steady flow of contagious entrants to the country. In short, a universal testing regime could quickly squelch the epidemic even if the test were of low sensitivity.
But there is a significant downside. Universal testing would generate enormous numbers of false positive results. With only a few thousand contagious individuals throughout the country on a given day, more than 37 million Canadians would be virus-free. Yet about three in a hundred—more than 1.1 million individuals—would (falsely) test positive using a test that has 97% specificity. Those individuals would have to quarantine for at least a day or two pending one or two subsequent negative tests to provide sufficient confidence they were not contagious. This would overwhelm confirmatory testing capacity and would be an enormous disruption to millions of Canadians and their employers. And since more than 99% of positive results would actually be false, many individuals would begin to disregard the results in view of the cost or inconvenience of a day or two in quarantine.[11]
It is completely unrealistic to contemplate daily or even weekly testing of all Canadians because nowhere near 37 million daily (or weekly) rapid PoC tests are likely to be available within planning horizons. For example, the US has contracted with Abbott Labs to eventually deliver 150 million antigen tests, but this would satisfy only four days of universal testing in Canada. Nevertheless, cheap and rapid tests can make an essential contribution to pandemic control provided a balance can be struck between (a) the benefit of detecting more asymptomatic and pre-symptomatic cases, and (b) the cost of very large numbers of false positives in situations where the prevalence of the virus is low.
3.3 Testing at Least Half a Million Canadians Every Day
What might be a practical strategy for testing much greater numbers of Canadians than at present so as to better control the spread of COVID-19 during the many months before an effective vaccine is available and widely administered? Development and implementation of such a strategy will also yield lessons for the control of future epidemics.
Currently, Canada is conducting about 75 thousand PCR tests per day with confirmed infections averaging 2.5%-3.0% of the total. If capacity were to be raised to 200,000 per day—a stated goal of the Public Health Agency of Canada—there would be considerable excess capability beyond the priority need for clinical diagnosis and contact tracing unless the current surge in infections fails to be brought under control. The question remains as to how much capacity might be needed to undertake the robust screening and reassurance testing that is necessary: (a) to control COVID-19 in view of its frequent asymptomatic and pre-symptomatic transmission; and (b) to create the public confidence that is needed to resume greater economic and social activity such as in-person schooling?
To illustrate the latter objective, consider international travel for business or tourism or other personal reasons. In 2019, there were more than 88 million crossings into Canada or 243,000 per day on average—37% of which were international and 63% were returning residents. Of the total, 4.5 million crossings were by commercial trucks entering from the US. In the month of June this year, the daily average number of all entrants was only 11,600, a reduction of more than 95% from a year earlier. (Trucking volume, which is dominated by the carriage of essential goods, was little changed from June 2019.) The decimation of international travel represents an enormous social and economic cost to Canada which could be substantially reduced if border restrictions, and particularly the two-week quarantine, were relaxed.[12] But to do so safely—particularly as long as COVID-19 is rampant in the United States—will require testing of entrants according to an assessment of the risk they pose. Assume for purposes of illustration that cross-border travel volumes were to resume to 75% of the 2019 average; that half of all arrivals were judged to be lower risk and tested once for reassurance; and that half were considered higher risk—e.g., because they are coming from an area of currently high infection—and tested twice for screening. The testing requirement under these assumptions would average about 270,000 per day. This hypothetical example, dealing with only one segment of the target population for screening/reassurance testing, illustrates the magnitude of additional capacity required, and also the payoff in economic and social terms if it were to be implemented.
Groups other than international travellers should also be subject to screening and/or reassurance testing as part of an integrated strategy to control COVID-19 within Canada. Table 3 below presents a very rough estimate of potential testing requirements drawing on approximate numbers of individuals in situations that merit testing—e.g., entrants to Canada, healthcare workers, residents and employees in long-term care facilities, students and their teachers, employees in a broad range of roles that require regular contact with the public (e.g., in retail, travel and hospitality), individuals in certain congregate settings known to be higher risk, and employees throughout the rest of the economy who may be tested for reassurance purposes. The numbers in the various target groups in the table are based on reliable sources but should be interpreted as order-of-magnitude estimates—refer to text following the table.[13]
The 3rd column in Table 3 (“average test frequency”) is very roughly indicative of potential test frequency averaged across the target group.[14] The actual frequency would vary significantly around the assumed average depending on local circumstances. For example, it is assumed in the table that the 760,000 persons employed in acute care hospitals, would on average be tested once a month thus generating about 25,000 tests per day. But the testing frequency might be much higher, at least for a time, if the facility were treating an elevated level of infection. This might be offset by less than monthly testing in hospitals in areas of very low incidence of COVID-19. Similar considerations apply to the other categories in the table. Specifically, because concentrated outbreaks (“super-spreader” events) are common with COVID-19, it would be effective to comprehensively test all those in the community, facility, or group associated with the outbreak. Use of an inexpensive rapid test greatly increases the feasibility of such limited population mass testing.
It is emphasized that Table 3 is intended only to illustrate potential requirements for Screening and Reassurance testing. (Canada’s current testing rate of roughly 75,000 per day is classified entirely as Diagnostic in the table, although many of these tests may actually belong more appropriately to the Screening category depending on the particular access protocols in various Provinces.) To meet the priority testing purposes of diagnosis and screening would, under the assumptions in Table 3, require national capacity on the order of at least 565,000 tests per day. Roughly 85% would likely be allocated to screening unless there were to be a severe outbreak, in which case the diagnostic requirements could increase significantly. But in that circumstance, cross-border travel would be severely curtailed, thus sharply reducing a particularly large generator of screening tests.
It is impossible in advance to estimate, even approximately, the likely demand for Reassurance testing. There have been examples in Ontario of people paying up to $400 for privately provided at-home testing. Clearly there is demand for reassurance testing but the volume will depend on the cost and availability of tests and on the perceived benefit in terms of worker and customer confidence and the peace of mind of individuals. Moreover reassurance testing is, by definition, of relatively less importance in a strategy to control the spread of COVID-19. We will not comment further on reassurance testing other than to point out that to optimize cost and convenience, it should primarily employ cheap and rapid (“RNX”) technology, and that the cost should be covered largely with private funds.
A rate of 565,000 tests per day for diagnostic and screening purposes would equate to testing approximately 1.5% of Canada’s population on average every day. A study sponsored by the Rockefeller Foundation called for a testing program in the US that would require 4.3 million tests per day.[15] On an equivalent per capita basis, the Rockefeller proposal would equate to almost 500,000 tests per day in Canada. This provides some further credence for the estimate in Table 3 and suggests that it is in the “ball park” regarding the total daily estimate despite substantial uncertainty as to allocation of the tests among various at-risk groups.
3.4 Scaling-up Canada’s testing capacity
The first priority for testing is clearly for diagnostic purposes, including contact tracing and associated testing. The federal Safe Restart program has earmarked $4.3 billion for the Provinces and Territories to support these purposes. That amount of incremental funding should be sufficient to cover 565,000 screening and diagnostic tests per day provided PCR tests are complemented with the new generation of rapid, inexpensive tests. Assume, for illustration, that 200,000 tests per day use the standard PCR technology—i.e. diagnostic tests, confirmatory tests, and some screening—and that 365,000 per day employ a more rapid, less expensive technology which would include, in addition to antigen tests, PCR tests optimized for cost-efficiency. What would be the approximate cost of the testing strategy outlined in Table 3?
An exceptionally well-documented recent study in the CMAJ estimated that the average all-in per test cost for screening with standard PCR technology was almost $120. However, by employing several efficiency measures—notably using saliva samples and pooling four samples at a time, among other techniques—it was estimated that the cost per test could be reduced by more than 40% to about $65. As for the cost of antigen testing, the US government has contracted for 150 million new antigen tests from Abbott Labs at US $5 per test. The Government of Canada has recently approved the Abbot Panbio antigen test and secured delivery of 8.5 million tests by year end, with an option to acquire a further 20 million in 2021. The cost has not been disclosed. Meanwhile, models exist for building highly efficient PCR labs that provide the ability to deliver mass testing at a contract cost that is becoming comparable to other rapid tests. This would be almost cost-competitive with antigen testing, with the advantage of higher sensitivity, although considerably longer turn-around time—e.g., 12-24 hours versus 15 minutes. There will nevertheless be many situations where a test-to-result time of 12-24 hours is quite acceptable, particularly to achieve higher sensitivity. The antigen and “efficient PCR” technologies are therefore complementary approaches to screening.
Returning to the diagnostic and screening testing volume of 565,000 per day estimated in Table 3, assume that: (i) 200,000 tests are performed in existing public sector labs at an average cost of $70 per test (for personnel, materials, and transportation), and (ii) 365,000 tests—some combination of antigen tests and the proposed private sector PCR network—are performed for an average cost of $20 per test.[16] The total monthly cost would be about $650 million of which perhaps two-thirds (covering tests performed in existing PCR labs) is already funded by the Safe Restart program. The cost of the proposed testing strategy needs to be assessed in the context of the alternative if COVID-19 were not controlled as efficiently as possible—i.e. cost of lost economic output; of lost years of life; of longer-term impacts on the mental and physical health of those infected. Larry Summers (a world-renowned economist and former US Treasury Secretary) and David Cutler have roughly estimated that the mass testing approach laid out in the Rockefeller Foundation study would produce a benefit-to-cost ratio of approximately 30 to 1.
In Canada, the key missing piece in the testing strategy advocated in this paper is the availability of very large numbers of cheap and rapid tests. The federal contract with Abbott Labs for antigen tests, as well as for a supply of ID NOW devices and test kits, is a good start. This should be complemented through support for the initial capital required to establish not-for-profit PCR labs to complement the rapid PoC technologies so as to achieve the large testing volumes required for Surveillance and Reassurance. Health Canada should accelerate approval of at least the rapid PoC tests that have already received the initial green light in those countries that have high-quality regulatory procedures. These tests, administered at scale and backed up by PCR, are essential tools to deal with the unique feature of SARS-CoV-2 spread—its pre-symptomatic and asymptomatic transmission.
. . .
ABOUT THE AUTHORS:
Vivek Goel - Special Advisor to the President and Provost at the University of Toronto and a Professor in the Institute of Health Policy, Management and Evaluation at the Dalla Lana School of Public Health. Professor Goel is a distinguished scholar with an extensive background in teaching, research and university administration. He obtained his medical degree from McGill University and an MSc in Community Health from U of T and an MS in Biostatistics from Harvard University School of Public Health. His research has focused on health services evaluation and the promotion of the use of research evidence in health decision-making. Professor Goel joined the University of Toronto in 1991 and went on to eventually serve as the University’s Vice President and Provost from 2004 until 2008. He was a founding scientist at the Institute for Clinical Evaluative Sciences (ICES), where he continues as an Adjunct Senior Scientist. He served as founding President and CEO of Public Health Ontario from 2008 until 2014, where he was highly successful in building an academic public health services agency that provided scientific and technical advice to front-line practitioners. He returned to the University of Toronto as Vice-President, Research and Innovation, and Strategic Initiatives and served in that role from 2015-2020. He has extensive experience in governance and serves on the boards of the Vector Institute, TRIUMF (Vice-Chair) and the Canadian Institute for Health Information (Vice-Chair). He is a member of the COVID-19 Immunity Task Force, the Governing Council for CanCOVID, the national research platform for COVID-19 research.
Peter Nicholson - Peter Nicholson has served in numerous posts in government, higher education, science, and business. His public service career included positions as head of policy in the Office of the Prime Minister, the Clifford Clark Visiting Economist in Finance Canada and Special Advisor to the Secretary-General of the Organization for Economic Cooperation and Development (OECD) in Paris. His business career included senior executive positions with Scotiabank in Toronto and BCE Inc. in Montreal. He retired in 2010 as the founding president of the Council of Canadian Academies, an organization created to support expert panels that assess the science relevant to issues of public importance. Peter Nicholson holds a PhD in Operations Research from Stanford University, has been awarded five honourary degrees, and is a member of both the Order of Canada and the Order of Nova Scotia. Fun Fact: Elon Musk credits Peter in his biography as giving him his first job.
Jeff Larsen - Educated in law and business, Jeff Larsen holds a BA from McMaster University, a Juris Doctor from the University of Toronto, a Master of Laws from Osgoode Hall Law School and an MBA from the Imperial College, University of London. Currently he is the Executive Director of Innovation and Entrepreneurship at Dalhousie University in Halifax and is also the Site Lead for the Creative Destruction Lab – Atlantic. He has also held senior positions in the investment sector as VP and General Counsel of Halifax-based Clarke Inc. and as Executive Director of Business Management and Chief Compliance Officer with CIBC Asset Management in Toronto. In keeping with his entrepreneurial and management experience with new businesses in the energy sector, Mr. Larsen is the co-founder of Seaforth Energy, Watts Wind and Katalyst Wind.
The views expressed belong to the author.
Read more opinion contributions via QUOTES from Air Quotes Media.